
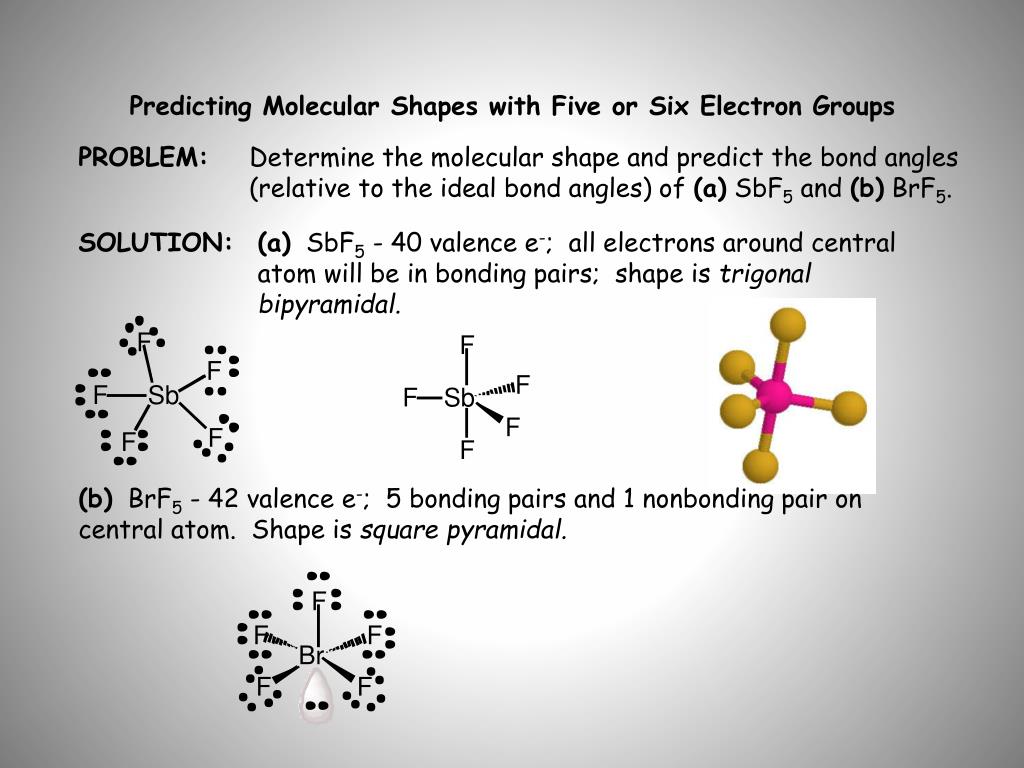

Now, after completing the octet of each participating fluorine atom, 40 valence electrons are exhausted in total. A single bond will be drawn between each Br-F interaction which means already 10 valence electrons out of 42 available are exhausted. When you begin drawing the structure, 5 fluorine atoms will be drawn around the central atom bromine. What is the anomaly in the Lewis dot structure of bromine pentafluoride (BrF5)? Step 5: Now draw the structure by assembling the above-mentioned points: If you realize the structure will be drawn properly which we will discuss now. Step 4: Look for the type of bond forming between the central atom and the others: Single covalent bonds are forming between the bromine and fluorine atoms. Step 3: Find the central atom in one BrF5 molecule: It will be bromine as the chemical element present as a single entity is considered as the central atom. Step 2: Find how many more valence electrons are required by one molecule of BrF5: It is 6 as one valence electron is required by each participating atom. Step 1: Find the total number of valence electrons one molecule of BrF5 has: It is 42 as 7 is coming from each of the fluorine and bromine atoms. Now, let us study the steps involved to draw the Lewis structure of BrF5. So, we need to add 2 electrons of the 2s and 5 electrons of the 2p to get a total of 7 valence electrons. The valence electrons are present on the highest principal energy level, the total number of them can be calculated by adding up electrons in the sublevels of those principal high energy levels. In the case of bromine, those are the 4s2 and 4p5 shells, so, the total number of valence electrons in bromine is 7.īesides this for Fluorine, its atomic number is 9 where its electronic configuration is 1s2 2s2 2p5. Here, it is important to understand that the electrons from the shells having the highest energy level, participate in the bond formation. The atomic number of Bromine is 35 where its electronic configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5.

To begin studying the Lewis structure of bromine pentafluoride, it is first crucial to study the Lewis diagrams of participating atoms. Conclusion Lewis Structure of Bromine pentafluoride (BrF5)


 0 kommentar(er)
0 kommentar(er)
